U.S. Antibiotic Awareness Week: Moving Forward from COVID-19
Each year, we observe U.S. Antibiotic Awareness Week (USAAW) and honor Lantana’s continued partnership with the Centers for Disease Control and Prevention (CDC). This year again we provide an update on the nation’s primary system for tracking inpatient antimicrobial use and resistance: the National Healthcare Safety Network (NHSN) Antimicrobial Use and Resistance (AUR) Module.
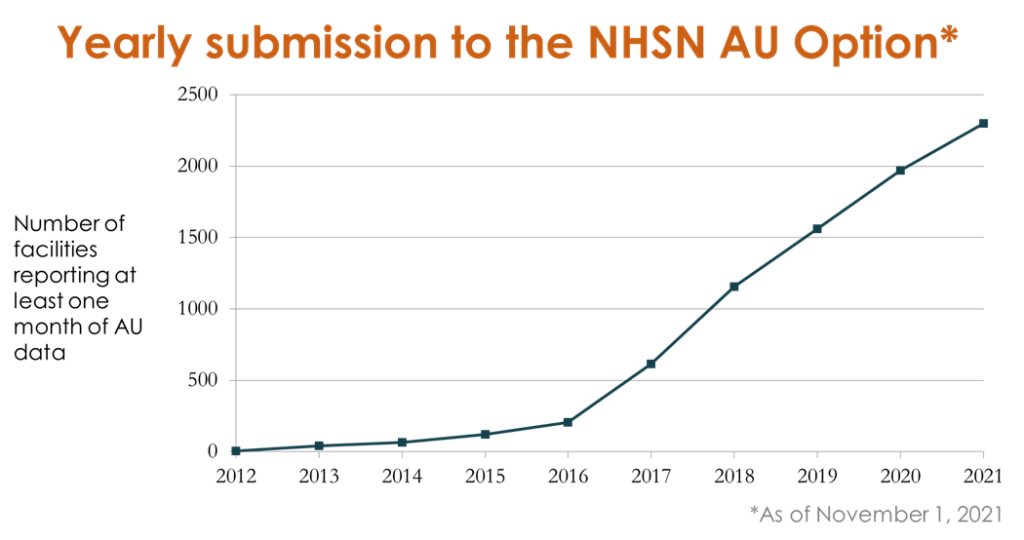
USAAW is an annual weeklong observance that raises awareness of the antimicrobial resistance threat and the importance of appropriate antimicrobial use. Antimicrobial resistance continues to threaten lives in the U.S. with more than 2.8 million antibiotic-resistant infections occurring each year resulting in over 35,000 deaths.1 CDC launched the NHSN Antimicrobial Use (AU) Option in 2011 to provide a mechanism for hospitals to report and analyze antimicrobial use. Through partner engagement, targeted education, and the release of a standardized risk-adjusted metric, AU Option enrollment and reporting has grown to nearly 2,300 hospitals voluntarily submitting AU data today. Noteworthy during a time of heightened awareness of the need for public health data automation, 100% of data submitted to the NHSN AUR Module is reported directly from electronic health systems using national interoperability standards. This process saves countless hours of healthcare and public health workers’ time when they’re already spread thin.
The COVID-19 pandemic overshadowed routine work at CDC in 2020 and 2021. Many members of the NHSN AU Option Team completed emergency rotations to aid CDC’s COVID-19 response. Heather Dubendris, a former AUR Team member, moved permanently to a position with the newly formed NHSN COVID-19 Vaccination Unit. Before she transitioned to her new role, she documented the AU Option’s response to the pandemic by capturing remdesivir’s use in hospitals.
In response to the antiviral remdesivir’s emergency authorization as a treatment for COVID-19 in May 2020, NHSN added remdesivir reporting to the AU Option and required hospitals to report its use beginning in July 2020. Heather and her colleagues on the NHSN AU Option Team found that 794 facilities (48% of facilities submitting AU Option data) reported remdesivir use in June 2020. Between July 2020 and May 2021, 1,561 facilities (86% of facilities submitting facility-wide inpatient data to the AU Option) reported remdesivir use at least once at the facility-wide inpatient level. Rates of remdesivir use were highest in respiratory critical care units and pulmonary wards. Heather presented these findings in a poster for the 2021 Council of State and Territorial Epidemiologists Annual Conference, titled Adapting during a Global Pandemic: Capturing Remdesivir in the NHSN AU Option.
Typically, NHSN makes decisions annually about which antimicrobials to add or remove from the AU Option. The mid-year addition of remdesivir and prompt uptake in reporting by hospitals participating in the AU Option allowed NHSN to track remdesivir use nationwide within a matter of months when data on the new treatment were most needed.
The AU Option Team will publish the 2020 AU Option Data Report following USAAW this year. The 2020 report, an update to the 2019 report, summarizes Standardized Antimicrobial Administration Ratio (SAAR) distributions and percentages of antimicrobial use for adult, pediatric, and neonatal locations. These reports provide actionable data to hospitals, informing stewardship by allowing hospitals to compare their SAARs to the national distribution and see where their prescribing practices can improve. The percentage of antimicrobial use by class and drug within SAAR antimicrobial agent categories included in the reports provides insight into prescribing practices across different patient care locations.

We cannot close out this blog without acknowledging CDC Surveillance Branch Chief Dr. Daniel Pollock’s retirement. Dr. Pollock spearheaded the creation of the AUR Module, and we owe so much of our success to his tenacity, leadership, and passion for antimicrobial use and resistance. He joined CDC as an Epidemic Intelligence Service officer in 1984 and retired in November of this year, leaving the Surveillance Branch and AUR Module in the capable hands of Dr. Andrea Benin and Dr. Hsiu Wu, respectively.
Congratulations, Dr. Pollock! Erin O’Leary said it best: Your passion is infectious! We promise to continue your legacy encouraging antimicrobial stewardship and protecting patients from antimicrobial resistant infections.
References
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed November 2, 2020.