Reporting Into the Antimicrobial Use and Resistance (AUR) Module
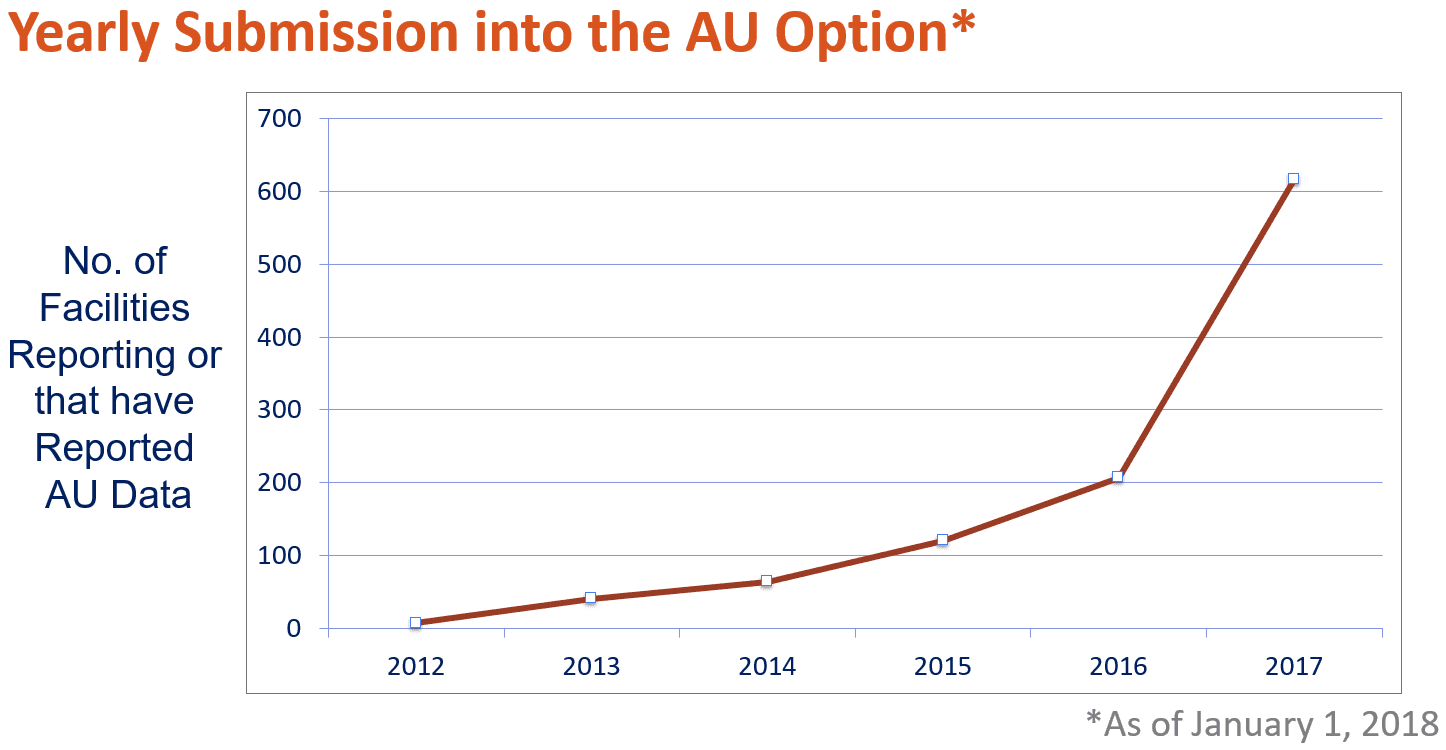
Our June 2017 blog on AUR (What is AUR and Why Do We Care?) introduced the most widely used healthcare-associated infection (HAI) tracking system in the nation—the Centers for Disease Control and Prevention’s (CDC’s) National Healthcare Safety Network (NHSN). Reporting into the Antimicrobial Use and Resistance (AUR) Module continues to increase. As of January 1, 2018, over 616 facilities from 48 states are reporting Antimicrobial Use (AU) data and over 231 facilities from 27 states submitted at least one Antimicrobial Resistance (AR) event or AR denominator. This is a 40% increase for hospitals reporting AU data and a 27% increase for AR data in the past six months.
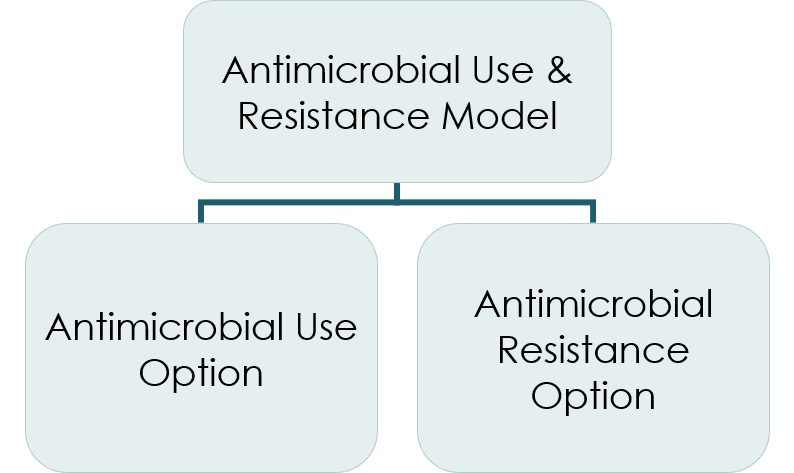
 Hospitals report and analyze AUR data via the AUR Module. The AUR Module covers two reporting options, one for antimicrobial use (AU) and one for antimicrobial resistance (AR). Facilities can participate in one (just AU or just AR) or both (AU and AR) at any given time.
Hospitals report and analyze AUR data via the AUR Module. The AUR Module covers two reporting options, one for antimicrobial use (AU) and one for antimicrobial resistance (AR). Facilities can participate in one (just AU or just AR) or both (AU and AR) at any given time.
To participate in either option, facilities must accurately capture the required data elements in one of their clinical information systems. The AU Option requires facilities implement either an electronic medication administration record (eMAR) or bar coding medication administration (BCMA) system in inpatient locations.
The AR Option requires facilities to use data directly from an electronic laboratory information system (LIS) or to extract the required data elements from another data source, such as an electronic health record (EHR). For both the AU and AR Options, facilities report patient care location information (e.g., type of ward or intensive care unit) as recorded in the facility’s Admission Discharge Transfer (ADT) system.
Facility personnel responsible for reporting AU or AR data must work with their software vendors to configure their system(s) to generate a standard formatted file to import into NHSN because there is no option to enter data manually into the AUR Module. All data must be submitted to the AUR Module via a Health Level Seven (HL7) format (i.e., Clinical Document Architecture (CDA)). As a first step, we recommend facilities contact their vendor to inquire about submitting AUR data. If the vendor cannot support AUR submission, the Society for Infectious Diseases Pharmacists website lists vendors who have attested to successfully reporting AUR data to NHSN. Some facilities leverage internal IT staff or informatics resources to report these data via homegrown systems. This requires specialized knowledge of coding and data aggregation and is not recommended for all facilities.
Challenges
AU and AR reporting calls for successfully completing a series of process steps:
- Location Mapping: associating the hospital’s specific ward and ICU patient care locations with NHSN’s generic terms for those locations
- Terminology Mapping: mapping local terms for antimicrobial agents and routes of administration to NHSN’s terminology
- Extract/Transform/Load AU data: establishing a stepwise process for culling AU data, transforming the data (as needed) to conform to NHSN requirements, and packaging the data in CDA files for upload or automated send to NHSN
- AU data validation and verification: assessing the accuracy and completeness of AU data extraction, transformation, and aggregation, and verifying that CDA files are created in accordance with NHSN specifications
- Maintenance: updating location and terminology mappings as needed and incorporating any changes in CDA specifications in file production process
Lantana staff, working closely with the CDC, provide technical support to assist facilities with AUR reporting. The Lantana team provides training and participates in meetings to encourage and guide AUR reporting for facilities of all sizes. They also develop training materials and guidance documentation. In addition, the team reviews the data submitted by facilities and provides facilities with feedback to promote valid data submission.
Lantana maintains the Lantana CDA Validator, where facilities can upload and test their CDA files against basic structural, syntactic, and semantic validation rules for NHSN submissions. If Validator detects an error, it provides feedback on the source and cause of the error. The NHSN AUR Module validates against additional business rules that are not detected by the Validator.
Facilities can choose to either submit the CDA files into NHSN or use vendor assistance to submit the CDA file via DIRECT CDA Automation, which allows vendors to directly submit the CDA files into NHSN on the facility’s behalf. This step eliminates the need for an NHSN facility user to login to NHSN to upload CDA files each month. Once implemented, AUR data submission via CDA is a streamlined reporting method leveraging AU and AR data that are widely available in electronic form. Lantana staff and the CDC recently developed a 12-minute Quick Learn video discussing uploading CDA files into NHSN help facilities with this process.
To connect with Lantana and CDC staff for technical assistance, please submit your AUR Module Protocol questions to the main NHSN CDC inbox: NHSN@cdc.gov. For technical CDA questions, email the NHSN CDA inbox: NHSNCDA@cdc.gov. Your inquiry will be routed to the appropriate staff members for response.
Additional Resources
For more information on NHSN, visit the NHSN website: https://www.cdc.gov/nhsn/index.html
For more information on the NHSN AUR Module, visit the NHSN AUR Module Protocol website: https://www.cdc.gov/nhsn/pdfs/pscmanual/11pscaurcurrent.pdf
For more information on NHSN and CDA, visit the NHSN CDA Submission Support Portal (CSSP): https://www.cdc.gov/nhsn/cdaportal/gettingstarted.html
