Electronic Attachments Tell a Comprehensive Health Story
Anticipated HHS regulations are expected to provide standards for electronic documents used as attachments to support reimbursement-related transactions. On February 5, I was joined by Mary Lynn Bushman, Sr. EDI Analyst at National Government Services, Inc., in presenting a HIMSS Health Story Project webinar on the attachment standards: their usage, the business case for their adoption, and their potential to move us closer to a patient-centered, longitudinal, electronic record—closer, that is, to a more complete “health story”.
Setting the Context for Standard Electronic Attachments
In a formal sense, attachments are defined as digital files delivered via a network, excluding paper, fax, and any physical media. Think of them as the “fax-killer” for healthcare. The anticipated new HHS rules apply to those attachment use cases described under HIPAA and the ACA, i.e., claims/reimbursement, prior authorization, referral, and audit. In plain language, for these use cases, an electronic attachment is everything you need to know that you don’t already have on hand. Health Story Roundtable participants are familiar with the story-telling power of standard electronic documents. The August 31, 2017 Roundtable explored how diverse document types—like the Discharge Summary, History & Physical, and Consult Note—supplement the ubiquitous Continuity of Care Document (CCD) to build towards a longitudinal record. The proposal on the table to fulfill the legislative mandate for standardizing electronic attachments builds on these existing, implemented standards, providing additional payback for their adoption.
Why Do We Need Them?
These attachments were long ago legislatively mandated—by HIPAA (1996) and, further, by the ACA (2010)—but never written into regulation. If we’ve gotten along this far without them, why do we need them, and why now? Health industry stakeholders have spoken out on these questions, forcefully and with stunning unanimity, in testimony to the National Committee on Vital Health Care Statistics. Their consensus view was subsequently echoed in an NCVHS letter to the HHS Secretary urging adoption of standards, with an incremental adoption plan, building on the standards already in use across clinical systems. And they said, “do it now.” A follow-up letter from NCVHS in mid-2017 reiterated this stance, emphasizing the cost savings of standardized electronic attachments. How much savings? The AMA has estimated between $21 billion and $210 billion or 10% to 14% of physician practice revenue on an annual basis is eaten up in inefficient claims processes, payment, and reconciliation alone. We have consensus on a direction, a sense of urgency, and belief that this could be the proverbial low-hanging fruit of health IT. And, with the publication last August of the required guidance from Health Level Seven (HL7), we also have a well-supported model for attachments standardization.
How Do We Use Them?
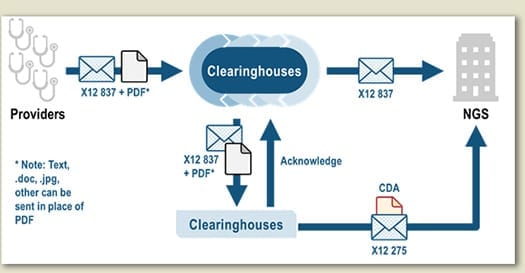
The simplest pattern for implementation is an unsolicited send where a claim transaction (X12 837) is sent along with the attachment transaction (X12 275) with the attachment inserted as a payload (HL7 CDA). Alternately, payers can request an attachment using an X12 277/278 transaction. The HL7 CDA document can be, of course, structured—i.e., any of the twelve types of structured documents cited as appropriate under Meaningful Use (per the 2.1 publication of C-CDA)—but can also—and this is critically important—be comprised of other existing or future forms of clinical documents, whether structured or not—i.e., the orphan document of the C-CDA, the one disallowed under MU, the unstructured document, and any newer type of CDA document that shares the metadata set defined by C-CDA.
While this builds on the same foundation as MU, the proposal is a game changer: it accommodates the fact that health records exist largely as unstructured notes. The expansion of what is allowable to an extensible set using a common metadata set (CDA “header”) and inclusion of simple, unstructured documents, and simple, un-coded XML documents, is a huge advance in building pathways to a comprehensive electronic record of care. Such simple CDAs can be produced through counter-top scanners that share context with a practice management system and simple XML CDAs can be delivered via any current dictation/transcription system. And of course, as structured documents from a EHR. Where does HL7 FHIR® come in? That remains for regulation to indicate. From a standards implementation perspective, it is an easy evolutionary path, both in terms of transactions which are being tested today in FHIR Connectathons and in terms of the payload where C-CDA-on-FHIR documents reiterate the functionality of C-CDA.
The Case of the Pilot that Never Stopped
At the Roundtable, Mary Lynn reported on a pilot begun over ten years ago between Mayo and an earlier Medicare support contractor, evolving later to a requirement when National Government Services (NGS) assumed the contract. Mayo sends around 3,000 unsolicited Operative Reports each year as simple, unsolicited XML CDA documents. Mayo likes the results: getting reimbursed faster (on average 30 days sooner than pre-pilot), fewer appeals, and less reliance on the US Postal Service—and payers appreciate the drop in appeals, denials, and call volumes. NGS likes it so much that they are expanding their implementation to work with other providers, this time in partnership with clearinghouses that can package the CDA for the provider as an added service. Quick to move through test and into implementation, that project is slowly ramping up and will soon be expanded to include solicited as well as unsolicited claims.
What does this mean for our Health Stories?
For providers, the main hurdles will be the knitting together of a comprehensive, indexed record bridging administrative and clinical systems. For payers and clearinghouses, there will be new transactions and data types. For the vendors supporting these stakeholders, there will be the challenge of indexing by document type code and validation against the required formatting rules. The opportunities are cost savings that reduce time to payment, reduce denials and appeals, reduce the cost of physical storage and materials, and reduce the labor involved in all the intensive handling that supports the system today. What is most striking about the projected financial impact of the new attachment standards is not only the aggregate savings but the manner in which they are balanced across stakeholders, especially at the low end of the investment scale—the transmission of simple, standard, electronic documents—where costs and benefits accrue to both providers and payers. What this means for our health stories goes beyond the immediate streamlining of payment transactions. A robust implementation of electronic attachments provides an avenue to ROI for a fully electronic and fully accessible patient record supporting care delivery, better health, better decision-making and creating a longitudinal record of lasting value, just as envisioned in The Health Story Project Values & Beliefs,.
